Updated 9 October 2023 (c) 2023
Introduction
In a separate article Radiometric dating), we sketched in some technical detail how dates are calculated using radiometric dating techniques. As we pointed out in that article, radiometric dates are based on known rates of radioactivity, a phenomenon that is rooted in fundamental laws of physics and follows simple mathematical formulas. Dating schemes based on rates of radioactivity have been refined and scrutinized for several decades. The latest high-tech equipment permits reliable results to be obtained even with microscopic samples.
Radiometric dating is self-checking, because the data (after certain preliminary calculations are made) are fitted to a straight line (an “isochron”) by means of standard linear regression methods of statistics. The slope of the line determines the date, and the closeness of fit is a measure of the statistical reliability of the resulting date. Technical details on how these dates are calculated are given in Radiometric dating. Here is one example of an isochron, based on measurements of basaltic meteorites (in this case the resulting date is 4.4 billion years) [Basaltic1981, pg. 938]:
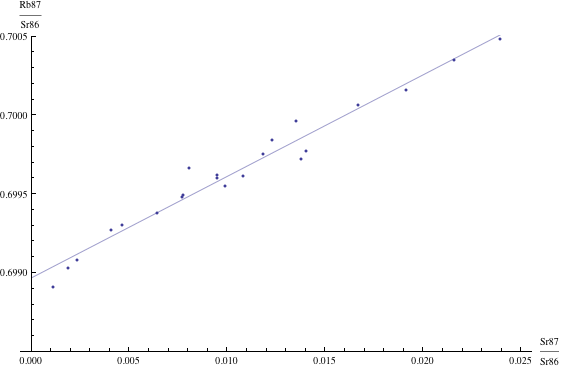
Reliability of radiometric dating
So, are radiometric methods foolproof? Just how reliable are these dates? The overall reliability of radiometric dating was addressed in some detail in a recent book by Brent Dalrymple, a premier expert in the field. He wrote [Dalrymple2004, pg. 80-81]:
These methods provide valid age data in most instances, although there is a small percentage of instances in which even these generally reliable methods yield incorrect results. Such failures may be due to laboratory errors (mistakes happen), unrecognized geologic factors (nature sometimes fools us), or misapplication of the techniques (no one is perfect).
We scientists who measure isotope ages do not rely entirely on the error estimates and the self-checking features of age diagnostic diagrams to evaluate the accuracy of radiometric ages. Whenever possible we design an age study to take advantage of other ways of checking the reliability of the age measurements. The simplest means is to repeat the analytical measurements in order to check for laboratory errors. Another method is to make age measurements on several samples from the same rock unit. This technique helps identify post-formation geologic disturbances because different minerals respond differently to heating and chemical changes. The isochron techniques are partly based on this principle.
The use of different dating methods on the same rock is an excellent way to check the accuracy of age results. If two or more radiometric clocks based on different elements and running at different rates give the same age, that’s powerful evidence that the ages are probably correct.
Rates of radioactivity
One question that sometimes arises here is how can scientists assume that rates of radioactivity have been constant over the great time spans involved. Creationist Henry Morris, for example, criticizes this type of “uniformitarian” assumption [Morris2000, pg. 91]. But numerous experiments have been conducted to detect any change in radioactivity as a result of chemical activity, exceedingly high heat, pressure, or magnetic field. None of these experiments has detected any significant deviation for any isotope used in geologic dating [Dalrymple1991, pg. 86-89; Dalrymple2004, pg. 58-60]. As another item of evidence, researchers studying a natural nuclear reactor in Africa have concluded that a certain key physical constant (“alpha”) has not changed measurably in hundreds of millions of years [Barrow2007, pg. 124-128]. Finally, researchers have just completed a study of the proton-electron mass ratio (approximately 1836.1526), and found that it has not varied more than 0.0005 percent over the history of the universe ranging back to 12.4 billion years ago [Srinivasan2016]. Thus scientists are on very solid ground in asserting that rates of radioactivity have been constant over geologic time.
Some young-earth creationists have also claimed that scientists cannot know with any certainty what happened millions of years ago — one would need a “time machine” for this. But in a curious twist of irony, scientists really do have time machines, in the form of astronomical telescopes. This is because when an astronomer views, say, a Type 1A supernova explosion in the Pinwheel Galaxy (see photo), which is known by completely different and highly reliable lines of evidence to be approximately 21 million light-years away, these astronomers are witnessing an event that transpired approximately 21 million years ago. And such observations have confirmed in exquisite detail that the physical laws that governed the universe 21 million years ago, specifically including the laws of quantum mechanics and radioactive decay that are relevant to geologic dating, are experimentally indistinguishable to those laws observed in laboratories today.

Anomalies
As with any experimental procedure in any field of science, these measurements are subject to certain “glitches” and “anomalies,” as noted in the literature. Skeptics of old-earth geology make great hay of these examples. It is true that some anomalies have been observed, but many of these claimed “anomalies” are completely irrelevant to the central issue of whether the Earth is many millions of years old. This is certainly true when errors are in the range of a few percent in specimens many millions of years old. This is also true of anomalies noted in carbon-14 dates, since carbon-14 dating cannot be used to reliably date anything older than about 50,000 years (because the carbon-14 half life is only 5730 years). For additional discussion, see
Radiocarbon dating.
Along this line, creationist Henry Morris [Morris2000, pg. 147] highlighted the fact that measurements of specimens from a 1801 lava flow near a volcano in Hualalai, Hawaii gave apparent ages (using the Potassium-Argon method) ranging from 160 million to 2.96 billion years, citing a 1968 study [Funkhouser1968]. In the particular case that Morris highlighted, the lava flow was unusual because it included numerous xenoliths (typically consisting of olivine, an iron-magnesium silicate material) that are foreign to the lava, having been carried from deep within the Earth but not completely melted in the lava. Also, as the authors of the 1968 article were careful to explain, xenoliths cannot be dated by the K-Ar method because of excess argon in bubbles trapped inside [Dalrymple2006]. Thus in this case, as in many others that have been raised by skeptics of old-earth geology, the “anomaly” is more imaginary than real. Other objections raised by creationists are addressed in [Dalrymple2006a].
Roger Wiens, a scientist at the Los Alamos National Laboratory, asks those who focus on anomalies or who are otherwise skeptical of radiometric dating to consider the following, among other facts [Wiens2002]:
- There are well over forty different radiometric dating methods, and scores of other methods such as tree rings and ice cores.
- All of the different dating methods agree–they agree a great majority of the time over millions of years of time. Some [skeptics] make it sound like there is a lot of disagreement, but this is not the case. The disagreement in values needed to support the position of young-earth proponents would require differences in age measured by orders of magnitude (e.g., factors of 10,000, 100,000, a million, or more). The differences actually found in the scientific literature are usually close to the margin of error, usually a few percent, not orders of magnitude!
- Vast amounts of data overwhelmingly favor an old Earth. Several hundred laboratories around the world are active in radiometric dating. Their results consistently agree with an old Earth. Over a thousand papers on radiometric dating were published in scientifically recognized journals in the last year, and hundreds of thousands of dates have been published in the last 50 years. Essentially all of these strongly favor an old Earth.
- A recent survey of the rubidium-strontium method found only about 30 cases, out of tens of thousands of published results, where a date determined using the proper procedures was subsequently found to be in error.
Additional details on these anomalies and other objections that have been raised by creationists are dealt with in detail in Roger Wiens’ article [Wiens2002], Mark Isaak’s book [Isaak2007, pg. 143-157] and a 2006 online article by Brent Dalrymple [Dalrymple2006]. A detailed response to other claims of scientific evidence for a young Earth is given by Matthew Tiscareno [Tiscareno2009].
Radioactive isotopes and the age of the Earth
As it turns out, there is a simple way to see that the Earth must be at least 2.72 billion years old, which does not require any mass spectrometers, isochron graphs, calculus or statistical software (provided one accepts a few very-well-established measured rates of radioactivity). Consider the list of all known radioactive isotopes with half-lives of at least one million years but less than one quadrillion years, and which are not themselves produced by any natural process such as radioactive decay or cosmic ray bombardment — see, for instance, the Wikipedia article “Nuclides” [Nuclides2022]:
| Isotope | Half-life (years) | Found in nature? |
| In-115 | 4.41 x 1014 | yes |
| Gd-152 | 1.08 x 1014 | yes |
| Ba-130 | 7.00 x 1013 | yes |
| Pt-190 | 6.50 x 1011 | yes |
| Sm-147 | 1.06 x 1011 | yes |
| La-138 | 1.02 x 1011 | yes |
| Rb-87 | 4.97 x 1010 | yes |
| Re-187 | 4.12 x 1010 | yes |
| Lu-176 | 3.76 x 1010 | yes |
| Th-232 | 1.40 x 1010 | yes |
| U-238 | 4.47 x 109 | yes |
| K-40 | 1.25 x 109 | yes |
| U-235 | 7.04 x 108 | yes |
| Pu-244 | 8.00 x 107 | yes |
| Sm-146 | 6.80 x 107 | yes |
| Nb-92 | 3.47 x 107 | no |
| Pb-205 | 1.73 x 107 | no |
| Cm-247 | 1.56 x 107 | no |
| Hf-182 | 8.90 x 106 | no |
| Pd-107 | 6.50 x 106 | no |
| Tc-98 | 4.20 x 106 | no |
| Bi-210 | 3.04 x 106 | no |
| Dy-154 | 3.00 x 106 | no |
| Fe-60 | 2.62 x 106 | no |
| Tc-97 | 2.60 x 106 | no |
| Cs-135 | 2.30 x 106 | no |
| Gd-150 | 1.79 x 106 | no |
| Zr-93 | 1.53 x 106 | no |
(In the above chart, years are displayed in scientific notation: i.e., 1 x 106 = 1 million; 1 x 109 = 1 billion, etc.)
All of the above isotopes are readily produced in nuclear reactors, so there is every reason to believe that they were formed along with stable isotopes, in roughly the same abundance as nearby stable isotopes of similar atomic weight, when the material forming our solar system was produced in an ancient stellar explosion. A quick calculation shows that after an elapsed period of 20 times the half-life of a given isotope, the fraction 1/220 = 1/1048576 (i.e., roughly one part in one million) of the original isotope will remain, which is a small but nonetheless detectable amount. Similarly, after 30 half-lives, roughly one part in one billion will remain, and after 40 half-lives, roughly one part in one trillion will remain, which is near the current limit of detectability.
Now note that an absolutely clear-cut fact is revealed in the above table: every isotope in the list with a half life less than 68 million years is absent in nature, evidently because all traces of these isotopes have decayed away, yet every isotope with a half life greater than 68 million years is present at some detectable level. This is incontestable evidence that the material from which our Earth and solar system was formed is at least 20 Sm-146 half-lives, i.e., 20 x 68 million (= 1.36 billion) years old, and, more likely, is at least 40 x 68 million (= 2.72 billion) years old. For details, see [Dalrymple2004, pg. 202-204; Miller1999, pg. 69-72].
Conclusion
In short, the geologic evidence for evolution, especially for the system of multimillion-year dates assigned to the various fossil layers and geologic epochs through the lifetime of planet Earth, is on extremely firm ground. To deny or blithely dismiss this conclusion, claiming instead that Earth was created a few thousand years ago, is tantamount to claiming that hundreds of thousands of peer-reviewed radiometric ages that have been published over the past 70 years, using numerous different dating techniques and approaches, are all simultaneously in error, not just by a few percent here and there, but instead by factors of thousands and millions.
Radiometric dating, like any other experimental discipline, is subject to a variety of errors, ranging from human errors to rare anomalies resulting from highly unusual natural circumstances. But while errors and anomalies can occur, the burden of proof is not on scientists to fully explain each and every error. Instead, the burden of proof is on skeptics of old-earth geology to explain why hundreds of thousands of other carefully measured ages are all internally and externally consistent.
The creationist-intelligent design approach of highlighting a few known faults and errors in past measurements is a classic instance of the “forest fallacy” — picking a fault or two in the bark of a single tree, then trying to claim that the entire forest doesn’t exist. But the forest does exist — hundreds of thousands of carefully peer-reviewed radiometric measurements have been made (and thousands more are published each year), using equipment and techniques that have been improved and refined over the past 70 years. This huge corpus of very well-established results cannot be easily dismissed, to say the least.
Indeed, there is no known physical phenomenon that can yield consistent results in many thousands of measurements, year after year, except one: that these specimens really are as old as the data shows them to be. As biologist Kenneth Miller has observed, “The consistency of [radiometric] data … is nothing short of stunning.” [Miller1999, pg. 76]. It is ironic that the one aspect of evolution that draws the most opposition, not only from the general public but also from the young-earth creationist community, namely the fact that Earth and its biosphere are vastly older than a few thousand years, is arguably the most experimentally solid of all.
For additional information on radiometric dating, including detailed responses to specific issues that have raised by creationists, see: [Dalrymple1991; Dalrymple2004; Dalrymple2006; Dalrymple2006a; Isaak2007, pg. 143-157; Miller1999, pg. 66-80; Stassen1998; Stassen2005; Wiens2002].
See also the articles on this website on radiometric dating (Radiometric dating) and radiocarbon dating (Radiocarbon dating).